Twelfth paper from a Short-Term Scientific Mission (STSM) (November 28, 2016)
In this work, the group of Prof. Edwin Otten (University of Groningen) reports the synthesis of an iron complex with formazanate ligands.
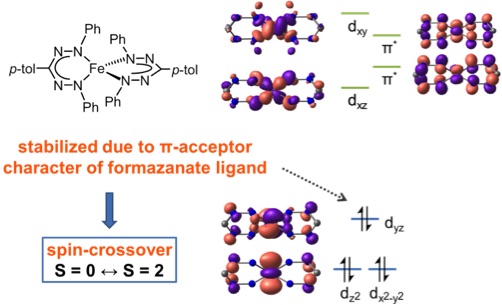
Surprisingly, this four-coordinate Fe(II) complex reversibly changes spin-state upon heating in solution from S = 0 (at low temperature) to S = 2.
X-ray structural analysis at low (100 K) and high temperature (450 K) indicate that spin-crossover also occurs in the solid state.
An S = 0 spin state is rare for four-coordinate Fe(II) compounds and in order to probe the reasons for this unusual behavior, a range of different spectroscopies was applied to study the system in detail.
In addition, DFT calculations were performed to obtain insight in the electronic structure.
It was shown that the formazanate ligands act as strong π-acceptors, which results in significant stabilization of one of the d-orbitals that normally has metal-ligand anti-bonding character in (pseudo)tetrahedral complexes.
As a consequence, the resulting d-orbital manifold resembles that found in octahedral symmetry ('3+2' splitting) and accounts for the unusual S = 0 spin state found experimentally for this d6 system.
In collaboration with the group of Prof. Franc Meyer (University of Göttingen), variable temperature UV-Vis and Mössbauer spectra were recorded on the system as well as magnetic (SQUID) measurements in the solution and solid state.
This work was carried out in the framework of an STSM by Raquel Travieso Puente, a PhD student at the University of Groningen (COST-STSM-CM1305-29908). The results of this collaboration were recently published as an Open-Access communication in JACS.