Fourteenth paper from a Short-Term Scientific Mission (STSM) (April 23, 2017)
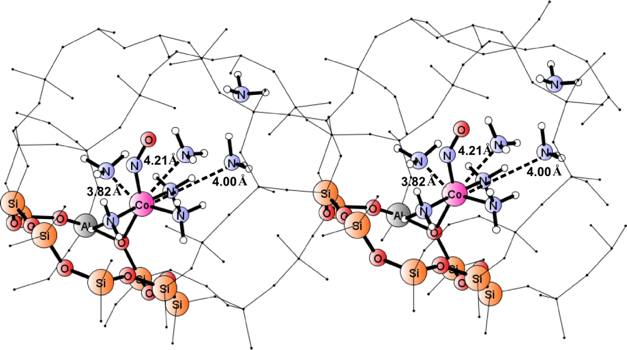
In this work, the group of Prof. Ewa Broclawik (Institute of Catalysis PAS) reports the study on the donor properties of cobalt-exchanged cationic sites in zeolites. It is based on cluster and periodic density functional theory modeling for relevant {[Co(II)(NH3)n]-NO} adducts, where Co(II) means a cobalt cation embedded either in a periodic model of chabasite (CHA) zeolite or in model clusters. NO stretching frequencies were derived from MD trajectories and compared to harmonic values from cluster calculations. By relating calculated NO frequencies to experimental FTIR spectra, it was shown that the {Co(II)-NO} adducts comprising three or four ammonia co-ligands in the singlet spin state dominate the spectrum taken in ammonia-saturation conditions while forms with two NH3 ligands, either in triplet or in singlet state prevail under intermediate ammonia saturation. Finally, this work confirms the critical dependence of Co(II) activation ability towards NO upon ligation of strong donor ligands, competing with electron-rich zeolite framework. Ammonia ligands not only reinforce donor properties of the center but also modulate its spin state.
In collaboration with the group of Prof. Tomas Bučko (Comenius University in Bratislava), Molecular Dynamics simulations for periodic models of zeolite frameworks was introduced and a procedure for calculating anharmonic frequencies from MD trajectory was incorporated. Introductory training in the methodology of periodic modeling was done in the group of Prof. Lubomir Benco (Vienna University). This work was carried out in the framework of two STSM missions by Adam Stępniewski, a PhD student at the Institute of Catalysis (COST-STSM-CM1305-21775 and COST-STSM-CM1305-21782). The results of this collaboration were recently published as an Open-Access paper in J. Mol. Model.