Fifteenth paper from a Short-Term Scientific Mission (STSM) (June 5, 2017)
The combination of high-resolution vibrational spectroscopy and spin-state consistent density functional theory has proven to be valuable for understanding transition-metal chemistry. In the collaboration between the groups of Prof. Browne (Univ. Groningen, NL) and Prof. Swart (Univ. Girona, ES), new insights were obtained
for a dinuclear nickel complex.
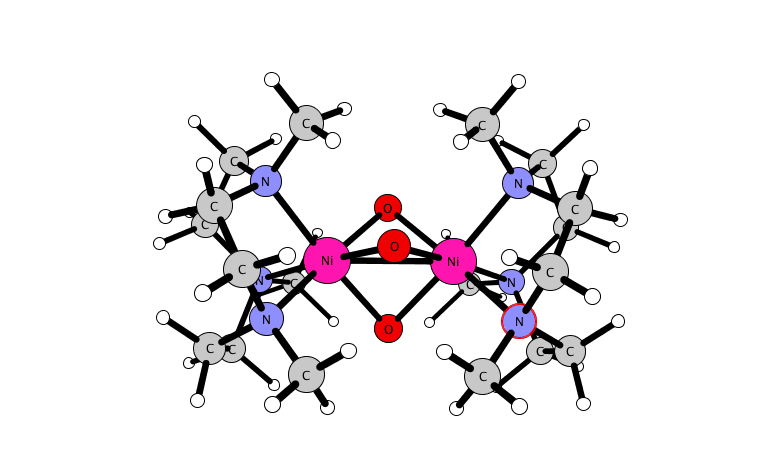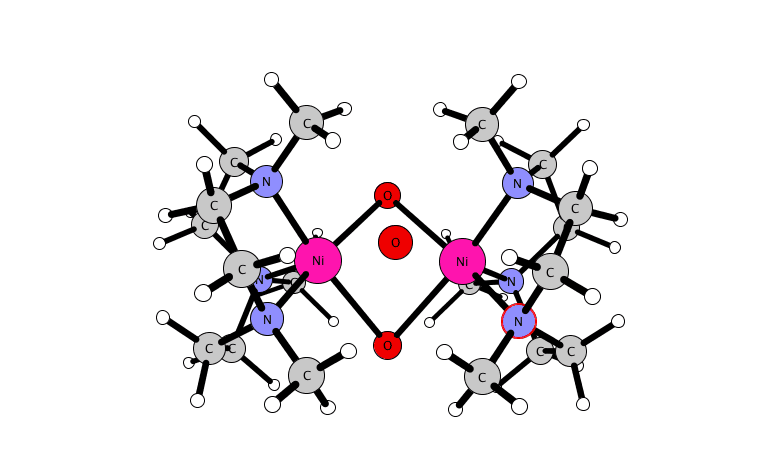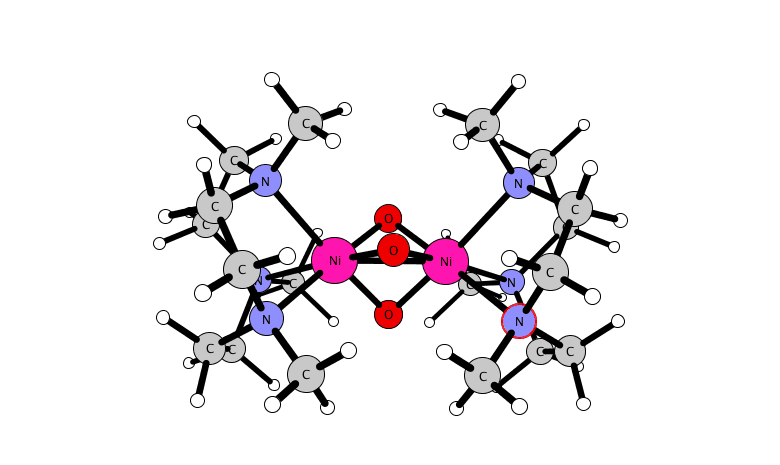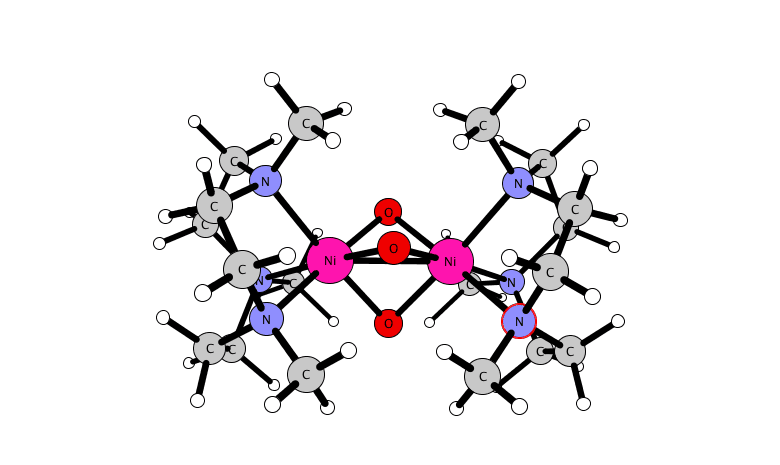
A reactive high-valent dinuclear nickel(IV) oxido bridged complex is reported that can be formed at room temperature by reaction of [(L)2Ni(II)2(μ-X)3]X (X = Cl or Br) with NaOCl in methanol or acetonitrile (where L = 1,4,7-trimethyl-1,4,7-triazacyclononane). The unusual Ni(IV) oxido species is stabilized within a dinuclear tris-μ-oxido-bridged structure as [(L)2Ni(IV)2(μ-O)3]2+.
Its structure and its reactivity with organic substrates are demonstrated through a combination of UV-vis absorption, resonance Raman, 1H NMR, EPR, and X-ray absorption (near-edge) spectroscopy, ESI mass spectrometry, and DFT methods. The identification of a Ni(IV)-O species opens opportunities to control the reactivity of NaOCl for selective oxidations.
The paper was recently published in Journal of the American Chemical Society.